Hardness is one measure of the strength of the mineral’s structure relative to the strength of its chemical bonds.
It is not the same as brittleness, which is another measure of strength, purely related to the structure of the mineral.
Minerals with small atoms, packed tightly together with strong covalent bonds throughout tend to be the hardest minerals.
The softest minerals usually have metallic bonds.
Hardness is generally consistent because the chemistry of minerals is generally consistent.
Hardness can be tested through scratching.
A scratch on a mineral is a groove produced by micro-fractures on the surface of the mineral.
It requires either the breaking of bonds or the displacement of atoms (as in the metallic bonded minerals).
A hard mineral can scratch a softer mineral, but a soft mineral can not scratch a harder mineral (no matter how hard you try).
Therefore, a relative scale can be established to account for the differences in hardness by merely seeing which mineral scratches another.
That is exactly what the German mineralogist Friedrich Mohs (1773-1839) proposed in 1812.
Below is the Mohs s hardness scale with some examples of different materials needed to affect each other.
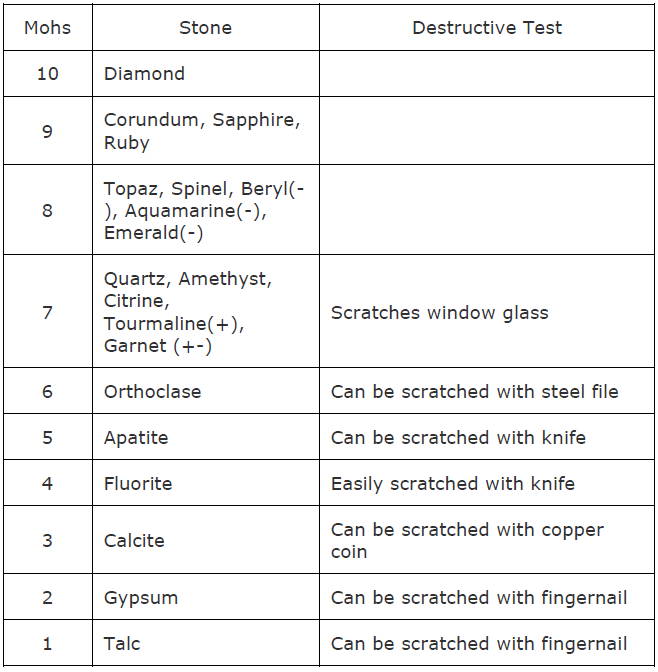
* Where a stone has (+) hardness can vary up to 0.5, eg. tourmaline has Moh’s hardness of 7-7.5
* Where a stone has (-) hardness can vary up to -0.5, eg. emerald has Moh’s hardness of 7.5-8
Below is a revised, more detailed Mohs hardness scale with some everyday items, crystals and metals listed:

Again, the Mohs Hardness Scale is only relative.
Meaning that fluorite at 4 is not twice as hard as gypsum at 2, nor is the difference between calcite and fluorite similar to the difference between corundum and diamond.
An absolute hardness scale looks a little different than the relative scale.
Using sensitive equipment, a comparison of the absolute hardness of minerals can be measured.
But as hardness increases, the difference in hardness significantly increases, as demonstrated below.
• 1 Talc
• 3 Gypsum
• 9 Calcite
• 21 Fluorite
• 48 Apatite
• 72 Orthoclase
• 100 Quartz
• 200 Topaz
• 400 Corundum
• 1600 Diamond
The simpler, relative Mohs hardness scale is much easier to remember and use.
It is easy to see why diamond gets so much respect as the hardest natural substance known to man.
The next hardest mineral, corundum, is four times softer!
Many substances are currently being created and studied to beat diamond in hardness.
But diamonds’ all carbon, incredibly dense, structurally sound and tightly bonded structure is hard to beat.
At present only diamonds created with isotopes of carbon have exceeded 10 on the hardness scale.
Hardness is particularly crucial for gemstones.
Tremendously few soft minerals are cut as gems, and when they are, they generally are cut only for collectors and not for wearable jewelry.
Most gemstones have a hardness of 7 or more.
Gemstones of hardness 6 or less are considered soft and are prone to easily scratching.
This is not to say such stones cannot be used in jewelry, but if worn, keep in mind that they must be handled carefully.
These stones can be damaged by dust, as dust contains small particles of quartz, which has a Mohs hardness of 7.
Hardness also plays a significant role in the minerals that are used for grinding, polishing, and other abrasive purposes.
Soft minerals can be used as high-temperature lubricants, pencil lead, talcum powder, paper gloss, etc.
Tenacity describes how the mineral behaves when subjected to deformation:
• Ductility – The subject can be drawn into thin wires.
• Elasticity/memory – The subject can be deformed by force but returns to its original shape when the deforming force is removed.
• Flexibility – The subject can be bent or deformed by force and remains deformed when the force is removed.
• Malleability – The subject can be flattened into sheets with hammerings, such as native gold, silver, and copper.
• Brittleness – The subject breaks or crumbles, which is the case for most stones and gems, but also for some metals that have been alloyed and or heat-treated (tungsten carbide, tempered steel, like files etc…).