If I say the word “ring,” the first image that will come to mind for most people will be yellow-colored.
Gold has been a metal of choice for jewelry for thousands of years… and still is today. The king of metals is seemingly irreplaceable, even though many other metals made their entry into the market in recent years.
But scientific research is opening doors not only to the introduction of other metals but also to exotic gold and inter-metallic gold alloys of incomparable beauty and charm, such as purple and blue gold, as well as new tarnish-free silver alloys, not to mention space-age materials like titanium, tungsten carbide, and the likes…
Arguably: there has never been a more interesting time in history to be a jeweler!
So keep on reading, and if you have any questions, please comment at the end of the article!
Gold has been treasured since ancient times for its beauty and permanence.
Most of the gold that is fabricated today goes into the manufacture of jewelry.
However, because of its superior electrical conductivity and resistance to corrosion and other desirable combinations of physical and chemical properties, gold also emerged in the late 20th century as an essential industrial metal.
Gold performs critical functions in computers, communications equipment, spacecraft, jet aircraft engines, and a host of other products.
Although gold is essential to industry and the arts, it also retains a unique status among commodities as a long-term store of value.
In the U.S., 10k is the legal minimum accepted standard of gold karatage, with 14k the most popular.
In most of the elder cultures and societies (like China, India, etc.), 18k is the lowest used standard to be called gold.
England accepts 9k gold, while in some countries, 8k is the legal minimum standard.
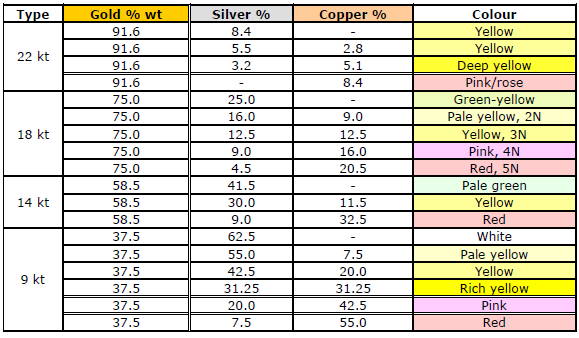
Here are the karatage equivalences in percentage:
24kt gold is pure .999 gold
22kt gold is 91.6 % gold
18kt gold is 75% gold
14kt gold is 58.5% gold
12kt gold is 50% gold
10kt gold is 41.7% gold
What mean “gold filled”?
Gold-filled is also called rolled-gold.
The jewels are made of base metal (usually brass or copper) covered by sheets of gold in the mechanical bonding process.
The karat gold content is typically 5% or 1/20 of the total weight.
Gold-filled is normally 10kt or higher karat over a base metal.
Traditionally made with 14k gold, it is hard-wearing.
With reasonable care, it will not peel or flake off and should last as long as solid 14k gold.
It is safe for most people with sensitive skin.
Gold-plated is a base metal that can be steel copper, silver brass etc. that is dipped into a bath of electroplating solution containing real karat gold.
When an electric current is applied, a thin layer of gold is deposited on the metal.
The gold plate is 10kt or higher.
The gold layer isn’t as thick as gold-filled and will wear off faster than gold filled.
24k gold reaches a hardness of 2.5 on the Mohs scale (talc 1, diamond10).

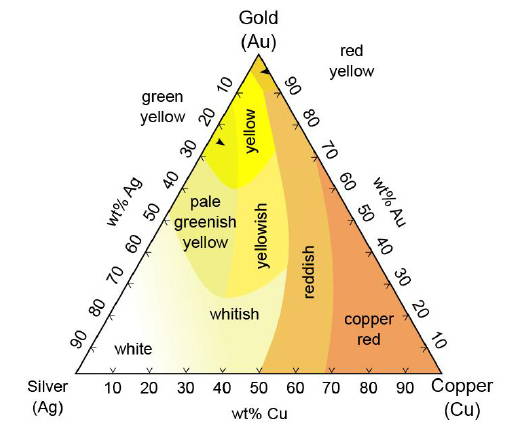
Almost all conventional, colored karat gold is based on gold-silver-copper alloys, often with minor alloying additions.
All three metals have the same crystal structure (face-centered cubic, FCC), and so are compatible with each other over a broad range of compositions.
Typical minor additions include deoxidizers such as zinc and silicon, grain refiners such as iridium and cobalt, and possibly metals such as nickel to strengthen the alloy.
Larger zinc additions (about 1-2%) can improve melt fluidity and hence ‘castability’ in lost wax casting, as can silicon, resulting in better filling of the mold and better reproduction of surface details.
Even larger zinc additions (up to 10%) can improve the malleability of certain karat gold, particularly 14 karats and lower ones, used to make jewelry by stamping from the sheet.
Additions of low melting point metals such as zinc, tin, cadmium, and indium are used to make carat gold soldering materials.

Gold is yellow, and copper is red: the only two colored pure metals.
All other metals are white or grey.
Adding copper to gold makes it redder, and adding silver, zinc, and any other metal makes gold paler.
So, the lower the karatage we plan to achieve, the more color options we will have.
Thus at 22 karats (91.6% gold), we can only add a maximum of 8.4% of alloying metals and hence can only obtain yellow to pink/rose shades.
At 18 karat (75.0% gold) and lower, we can add 25% alloying metals and hence get colors ranging from green-yellow to yellow to red, depending on the copper-silver/zinc ratio.
Thus at any given karatage, we can play with the color intensity by changing the copper-silver/zinc ratio.

This can be demonstrated in the following table:
White gold is an alloy of yellow gold with other white metals, such as zinc, nickel, palladium, and silver.
White gold is durable and resistant to tarnishing and fire scale, but it can be brittle and requires rhodium plating to get a true white result.
Some alloys will give whiter colors than others, but none of them is really as white as rhodium.
Once the plating wears off, it can cause allergic reactions if it contains too much nickel: these nickel alloys are now banned for use in many countries.
It was originally produced to be a more cost-effective substitute to platinum, even though the shifting of prices for the 2 noble metals in recent years made this reason less prevalent.
Rose (pink) Gold is alloy copper with gold, and it was first discovered in Russia, hence the name it still bears today: Russian gold.
By altering the different percentages of each, the intensity of rose gold will be lighter or darker.
Because of the copper content, rose gold can sometimes slightly darken over time: this is mostly due to the chemical composition of the user’s sweat and his/her lifestyle and occupation.

Green gold is achieved when silver, and sometimes silver mixed with some small amounts of copper and zinc or cadmium (baned since cadmium creates poisonous fumes when molten), are used as the alloys.

Intermetallic gold alloys:
Purple gold is also referred to as amethyst or violet gold.
The process of making purple gold is to alloy gold and aluminum at a ratio of 80% gold and 20% aluminum.
Cast pieces of purple gold are sometimes machined and faceted to be used as ‘pseudo gems’ in jewelry, as this alloy is otherwise not easy to process, and even impossible to laminate or pull, due to its high degree of brittleness.

Blue Gold is also made as an intermetallic compound between gold and indium, and sometimes even iron.
Manufacturing techniques are similar to those for purple gold.
Both alloys are described as quite brittle and hard to process.

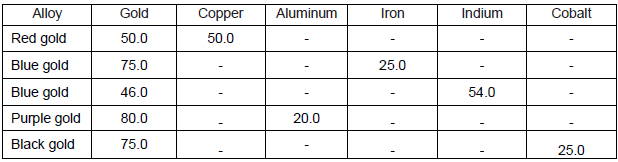
Above is the composition of different intermetallic gold alloys.
Purple gold and blue gold (alloyed iron gold) are prone to discoloration since the alloys giving them their color can be attacked by oxygen.

Plating
In the quest for different colors, another technique is used, which involves the deposition of metal of different colors on top of the base metal.
Brown, black (rhodium), and blue (rhodium) colors can thus be achieved.

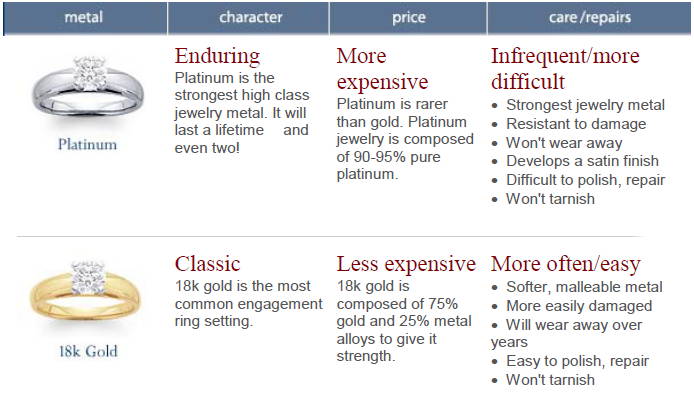
Platinum is malleable, ductile, precious, hypoallergenic gray-white metal, highly resistant to corrosion.
It does not tarnish or fire scale, but casting and soldering can be tricky because of its high melting temperature.
Platinum is scarce, which accounts for its high market price.
Unlike gold, it is used in jewelry in almost its pure form (typically 90% or 95% pure).
It’s an extraordinarily long-wearing and very white metal, so it does not need to be rhodium plated like white gold does.
Platinum’s natural white luster provides a rich backdrop for diamonds, but it’s a metal that’s as elegant when used all by itself to create a piece of jewelry, either a simple polished item or a design with engraved motifs.
It looks stunning when combined with contrasting touches of 18K yellow gold.
Six related metals belong to the Platinum Group of Metals or PGM:
-Platinum
-Iridium
-Palladium
-Ruthenium
-Rhodium
-Osmium
Jewelry that contains 850, 900 or 950 parts per thousand of pure platinum may be marked “Plat” or “Pt” if a number is used in front of the term to disclose the amount of pure platinum in the mix, such as:
-“850 Plat” or “850 Pt”, or
-“950 Plat” or “950 Pt”
Jewelry that contains at least 950 parts per thousand of platinum group metals, with at least 500 parts per thousand of the total pure platinum, may be marked as platinum as long as the numbers of each metals are disclosed.
For instance,
-“600 Pt. 350 Ir.” or 600 Plat. 350 Irid.” for 600 parts pure platinum
and 350 parts iridium
-“550Pt. 350Pd. 50Ir.” or “550Plat. 350Pall. 50Irid.” for 550 parts pure
platinum, 350 parts palladium and 50 parts iridium
Platinum reaches a hardness of 4.25 on the Mohs scale (talc 1, diamond 10).

Palladium is a rare silver-white metal of the platinum group.
Palladium resembles platinum chemically and is primarily used as an industrial catalyst and in jewelry.
It is gaining popularity in the world of jewelry.
It is as white as platinum, harder than gold, and it weighs less than platinum.
Palladium will not tarnish.
Palladium reaches a hardness of 4.75 on the Mohs scale (talc 1, diamond 10).

Rhodium is a rare silvery-white hard metal.
Rhodium is a member of the platinum group.
It is the most expensive precious metal, very similar to platinum, and sharing many of the properties of platinum, including its white color.
Rhodium plating is used to make white gold look whiter since the natural color is slightly yellowish-grey.
Rhodium plating is very white and very hard, but it does wear away eventually.
Rhodium reaches a hardness of 6 on the Mohs scale (talc 1, diamond 10).

Fine silver is 999 parts out of 1000, which is generally very soft and easily bent.
Sterling silver is 925 parts of silver and 75 parts of the alloy (usually copper).
These are the proportions the metal should have to be called “sterling silver.”
Because of the addition of copper, sterling silver will tarnish and is prone to a fire scale.
However, it is the copper alloy that gives strength to the sterling silver.
Silverplate is a thin layer of fine silver over a base metal that wears off easily.
Fine silver reaches a hardness of 2.5 on the Mohs scale (talc 1, diamond 10).

Titanium is a natural element that has a gray-white color.
Titanium is one of the strongest unalloyed metal in the world.
It is three times the strength of steel and much stronger than gold, silver, and platinum, but is very lightweight.
Pure titanium is also 100% hypoallergenic, which means that it is safe for anyone to wear.
Interesting fact: its surface (which is called titanium oxide) can take virtually any color with proper use of the anodizing process.
Many kinds of titanium alloys can be found on the market, though, and all of them have different properties (incredible strength, superior resistance to various acids or environments, more ductility, etc…).
Titanium reaches a hardness of 6 on the Mohs scale (talc 1, diamond10).

Tungsten carbide often referred to as the hardest metal, has a higher compressive strength than any other metal or alloy,
including steel.
Because it is highly resistant to abrasion, it is ideal for use in various wear applications.
It has begun to make its way in the jewelry market only recently (more or less 20 years ago).
Even if it can be quite brittle (a good slap on a stone tabletop can split a tungsten carbide ring in half), some people enjoy wearing that high tech metal for its weight (almost the same as platinum!) and its incredible resistance to surface damage.
When well polished, it has a beautiful shiny, grey luster.
Tungsten carbide reaches a hardness of 9 on the Mohs scale (talc 1, diamond 10).

A cheap but interesting alternative to traditional jewelry metals, stainless steel 316 is the highest grade of stainless steel on the market.
It is also called surgical steel because it’s widely used in the health care sector for tools and even implants.
This alloy has a very high resistance to acids and various environments, has a moderate weight, is quite strong, and have a nice nearly white luster.

Copper is a reddish gold metal that patinas to a warm brown but can also take on a nice green patina when exposed to outdoor environments.
Copper and gold are the only two naturally colored metal.
Copper reaches a hardness of 3 on the Mohs scale (talc 1, diamond 10).

Brass is a copper and zinc alloy.
It will tarnish and turn brown over time.
Brass reaches a hardness of 3.5 on the Mohs scale (talc 1, diamond 10).

Argentium silver, invented in 1996 by Peter Johns, like sterling silver, is at least .925 pure silver.
Unlike sterling, which is 0.75 alloy copper, argentium has a small amount of germanium instead of copper.
It is free from tarnish and fire scale, more malleable, easily fused, and fired with metal clay.
Germanium is an element similar to tin and silicon.
Platinum silver was created by American Bullion Precious Metals in Carson, CA, in 2003.
It has three formulas, all beginning with .925 sterling and 1%, 3.5%, or 5% platinum.
This metal is a cheaper alternative to white gold.
This alloy can be fabricated and cast more easily than platinum and is highly tarnish resistant.
Precious Metal Clay PMC is a metal compound developed in the early 1990s in Japan by metallurgist Dr. A. Morikawa.
The material consists of extremely fine precious metal powder in a colloidal suspension, which burns off on firing.
Success was first achieved with gold and later duplicated with silver.
The material is modeled into the desired shape and then kiln-fired: the binder then burns away, leaving just the precious metal, and the object is smaller because the binder has been removed.
Shrinkage from 8 to 20% occurs (depending on the variety used).
Another brand, Art Clay Silver (ACS), has similar properties.